This post is also available in Dutch .
Scientists know how smell works in the brain but not exactly how neurons “read out” chemical information. Quantum biology could provide an answer.
What happens when you smell something? Chemicals, the odour molecules, travel into the nose and bind to receptors located in the olfactory epithelium (the surface in the nasal cavity). This binding triggers a neural signal that travels to the olfactory bulb first, and from there, to other brain areas, including those implicated in emotional reactions and memory. This latter part, how smell works in the brain (the neural signalling) is well-understood. What is unclear is how binding occurs.
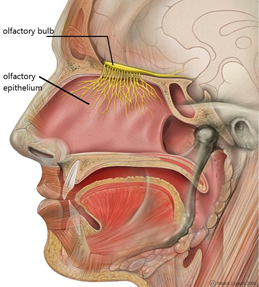
A well-accepted theory claims that the odour molecule has a particular shape that allows it to fit in the receptors in our nose. The receptor and the molecule are like a lock and a key. If the shape is not “right”, it will not fit into the receptor. When the shape does fit the receptor, it triggers a neural signal and so a unique sensation of smell.
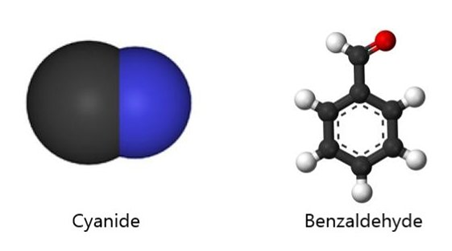
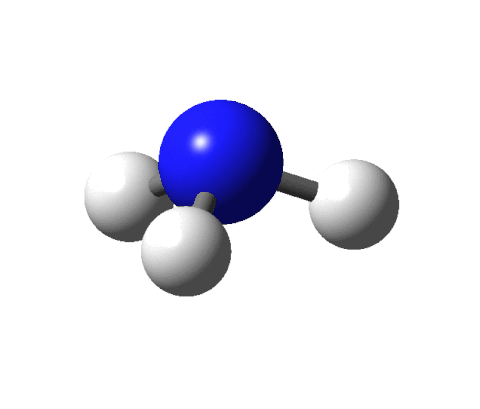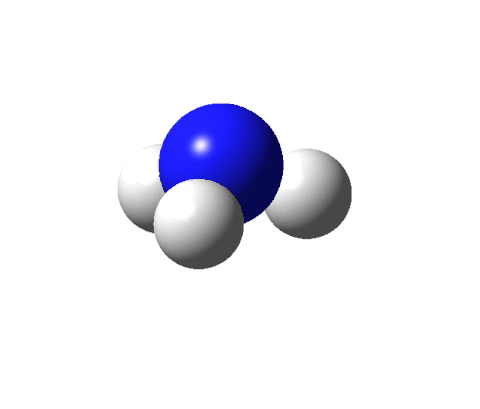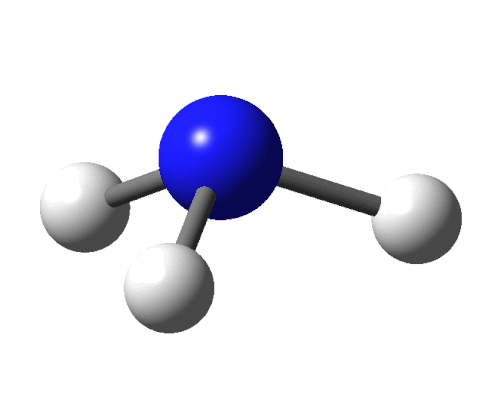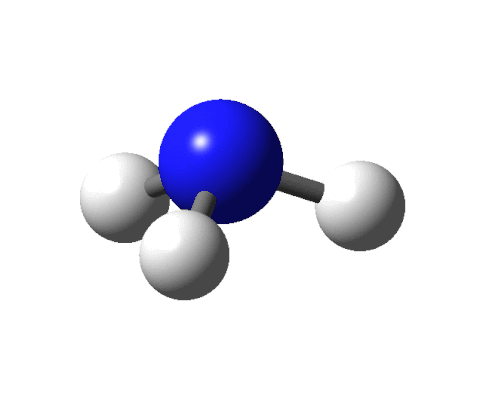
This sounds like an elegant idea except for a couple of things. There are fewer receptors (around 400) in our noses than the molecules that we can smell (around 10,000). Moreover, there are odour molecules that smell the same but have different shapes. To give an example, benzaldehyde and cyanide are shaped differently yet both smell of almonds.
The lock-and-key mechanism is probably not the whole story of how the binding of molecules to receptors works. Some scientists say that processes from quantum mechanics can explain why molecules with different shapes can smell the same. The idea is that receptors can sense vibrations of the odour molecule.
Atoms in a molecule are held together by bonds that resemble strings. These strings vibrate. The receptor contains quantum particles (electrons). Vibrations change the distance between the odour molecule and the walls of the cavity in which it sits. This helps the electrons to hop from one side of the receptor to the other side (to tunnel). This electric current is then what the receptor senses to trigger a smell response. But as electrons leap from one atom to another (before tunneling happens) they destroy the molecule’s electric field. This is like plucking a string. So in addition to molecule’s bond vibrations, the electrons too, vibrate the bonds in a molecule before they tunnel.
Animal research confirms that some animals, such as fruitflies can smell atom vibrations. In a study from 2011, researchers took two molecules, one containing ordinary hydrogen and the other, deuterium. The molecules had the same shape. The only difference was that deuterium is heavier and so it vibrates more slowly than ordinary hydrogen. If atom vibrations did not matter for recognition, fruitflies should not be able to tell the difference between the two molecules. However, the opposite happened.
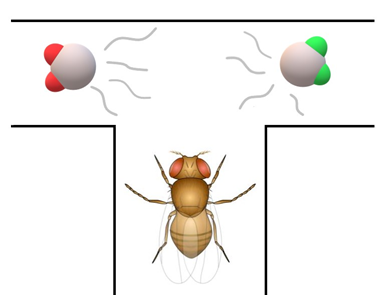
Researchers trained flies to avoid the deuterated version of the molecule. The training was done by using mild electric shocks. Then, scientists had fruitflies pass through a T-shaped maze. They could go for deuterium (that was conditioned to be avoided) or ordinary hydrogen. The flies preferred the latter. Learning could only happen if flies could tell the difference between the two molecules. Interestingly, fruitflies additionally avoided a different (undeuterated) molecule that had a similar range of vibrations as deuterium. This confirms that fruitflies are sensitive to this frequency information. As for us, humans, experiments have been conducted but results are inconclusive.
It can be concluded that smelling is similar to hearing because waves get picked up. Although vibrational theory is not mainstream science, it deserves to be taken seriously…. if a more complete story of how smell works is to be proposed.
Original language: English
Author: Julija Vaitonyte
Buddy: Floortje Bouwkamp
Editor: Ellen Lommerse
Translator: Jill Naaijen
Editor Translation: Wessel Hieselaar
Image credits
Pixabay (license)
Ball-and-stick model of the cyanide anion by Ben Mills via Wikimedia Commons
Ball-and-stick model of the benzaldehyde molecule by Jynto and Ben Mills via Wikimedia Commons
Giphy
Fruitfly (Drosophila melanogaster) from Database Center for Life Science (DBCLS) via Wikimedia Commons
